What is galvanized vs. zinc plating?
Both galvanized and zinc plating are a form of galvanization in which a protective zinc coating is applied to steel or iron to prevent rusting. In this article, we will cover the basic differences between the two. It is good to understand the differences when selecting a product. Often one assumes that the term ‘galvanized’ means the product is ‘hot-dipped galvanized’ when it could very well be electro-galvanized, which is simply another term for zinc plating. A big difference is the coating thickness. Zinc plating is normally 0.2 mils thick, whereas hot dip galvanizing may be up to 1.0 mil thick, providing 5 times the protection.
Hot-Dip Galvanizing
Hot-dip galvanizing is the most common method, in which the metal parts are fully submerged in a bath of molten zinc. The hot-dip galvanizing process has several steps including degreasing, rinsing, pickling, rinsing, flux solution, drying, zinc bath, cooling, and inspection.
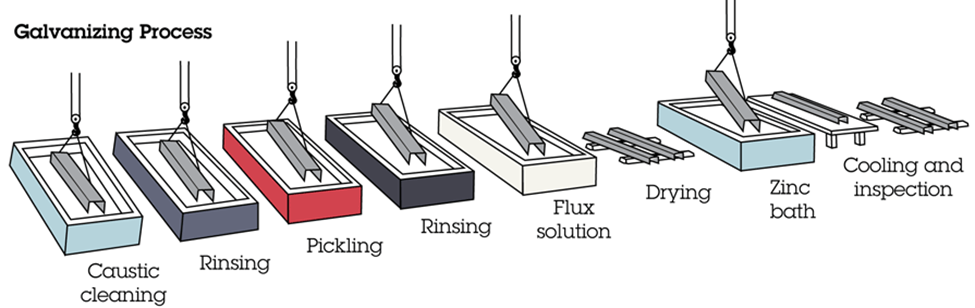
Hot-dip galvanizing is commonly used for pipe fittings, larger parts such as tanks and pressure tanks, fencing and gates, outdoor stairs and railings, structural beams, telecommunication towers, and much more. The steel is dipped into a big bath, where the coating is applied. The finish will be somewhat rough and may have runs in the plating. It will be a dull grey. Hot-dip galvanized products make up for their poor looking finish by providing superior protection.
Due to the thick coating, hot-dip galvanizing is not suitable for all types of parts and therefore is not used on components with threads or small profiles which may be filled with plating making it impossible to make proper joints, for example. This is why you will notice that hot-dipped galvanized pipe fittings and pipe nipples have the threads cut after the hot-dip process. This unfortunately leaves some of the uncoated metal exposed.

Zinc Plating / Electro Galvanizing
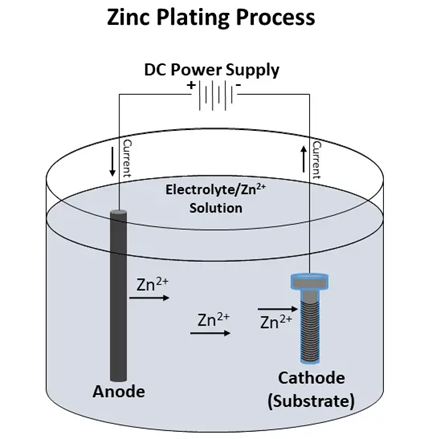 Sometimes referred to as electro-galvanizing, the zinc plating process applies zinc using an electrical current. Immersed in a solution, the desired part/component acts as a cathode and the surface coating agent as an anode. An anode-cathode reaction occurs when the power is applied, which dissolves the zinc in the anode. The zinc then migrates through the liquid to the cathode, and thus creates a thin layer of zinc on the surface of the workpiece.
Sometimes referred to as electro-galvanizing, the zinc plating process applies zinc using an electrical current. Immersed in a solution, the desired part/component acts as a cathode and the surface coating agent as an anode. An anode-cathode reaction occurs when the power is applied, which dissolves the zinc in the anode. The zinc then migrates through the liquid to the cathode, and thus creates a thin layer of zinc on the surface of the workpiece.
The thinner coating does not provide the same level of rust resistance as hot-dip galvanizing. However, the main advantage is that the plating thickness is thinner and can be better controlled. Therefore, it is ideal for threaded plumbing components which can be zinc plated after the threads or other profiles, such as insert barbs, have been machined. This is a great advantage since there is no bare metal left exposed to the fluid media or elements. In addition, the product will have a very good looking, shiny, and smooth finish.
Zinc plating is commonly used for smaller parts such as steel nuts and bolts and insert fittings for the plumbing industry.

Each method has its advantages and disadvantages however, depending on the application one method may be much more suitable than the other.
